This means single replacement reactions are by definition oxidation-reduction reactions. Single replacement reaction displacement.

Single Replacement Single Displacement Reaction
The reaction takes place when different types of metals or halogens come into contact with one another such as zinc dissolving in an acidic solution.

. Single replacement reactions are chemical reactions where in some cases metals REPLACE other metals that are in a solution. Single Replacement Reactions 1. This indicates how strong in your memory this concept is.
A single-replacement reaction A chemical reaction in which one element is substituted for another element in a compound. A single displacement reaction is a specific type of oxidation-reduction reaction. In a double replacement reaction there is interchanging of ions or atoms between the reactants.
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. Definition of single replacement or single displacement reactions. There are two types of single replacement reactions.
Zn CuCl_2 Cu ZnCl_2 A halogen replaces another halogen that is in solution. The reactivity order corresponds to the reactivity series of the metals. For example 2 HClaq Zns ZnCl 2 aq H 2 g.
In a single replacement reaction a single element replaces an atom in a compound producing a new compound and a pure element. A BC B AC Example. Cationic exchange or anionic exchange.
Single Replacement Reactions 2. Neutralization precipitation and gas formation are types of double. In single replacement reactions one ion or an atom of an element is displaced by another ion or atom discussed in detail below.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. This indicates how strong in your memory this concept is.
A single replacement reaction occurs when an element reacts with a compound displacing another element in that compound according to the University of Memphis. Think about it like these people. A single-displacement reaction also called single-replacement reaction is a type of oxidation-reduction chemical reaction when an element or ion moves out of one compound and into another.
Chemical reaction involving ions where one element is replaced by another in a compound. A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
A substitution or single displacement reaction is characterized by one element being displaced from a compound by another element. Is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a. Single replacement or displacement reactions occur when an element most frequently a metal replaces another element from a compound.
Predicting and determining the products using the reactivity series. Alright so were going to talk about single replacement reactions you might also. Orange shirt guy is by himself.
The reactivity order corresponds to the reactivity series of the metals. They can occur in two forms. A single-replacement reaction sometimes referred to as a single-displacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products.
A metal replaces another metal that is in solution. Chemical reaction involving ions where one element is replaced by another in a compound. To drive this point home well assign oxidation states in some of our examples to follow the flow of electrons in the reaction.
A single replacement reaction aka single displacement reaction will occur if M 1 cation is less reactive than M 2. Like double replacement reactions metals always replace metals and nonmetals always replace nonmetals in a compound.
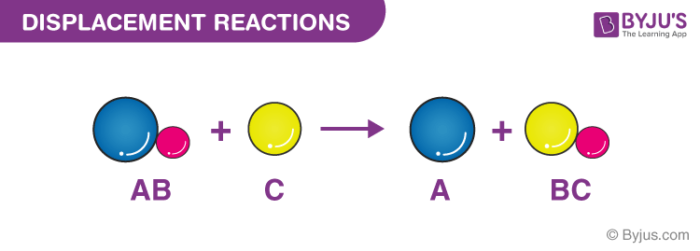
Displacement Reactions Definition Types Single Double Examples

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Replacement Reaction Definition And Examples
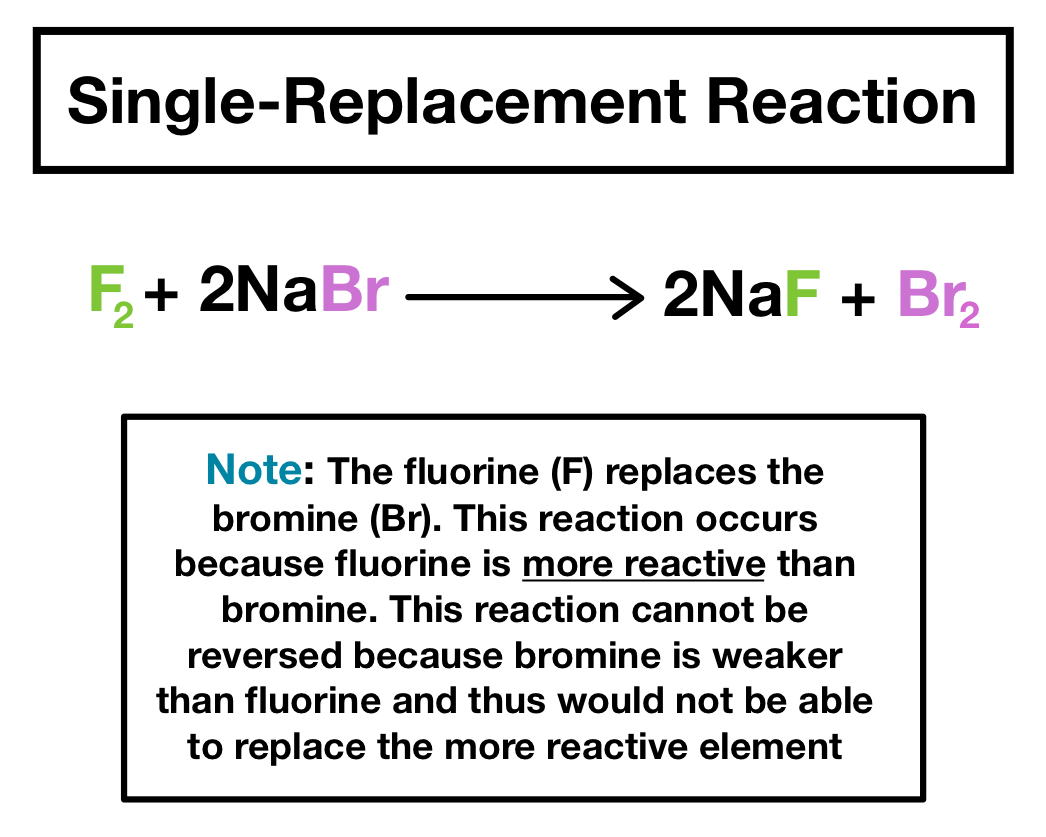
Single Replacement Reactions Definition Examples Expii
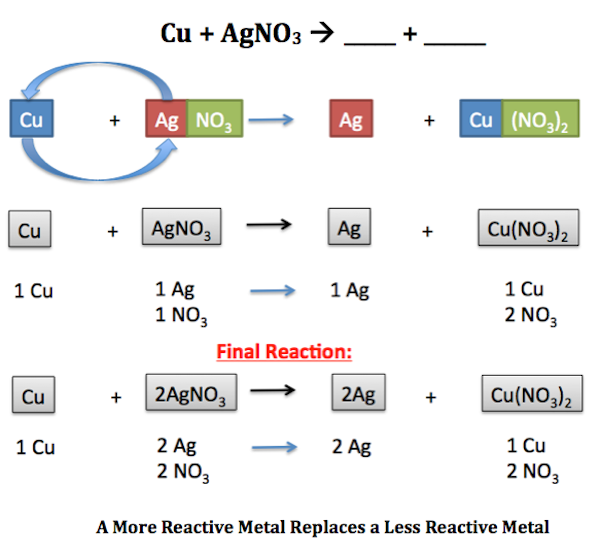
Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
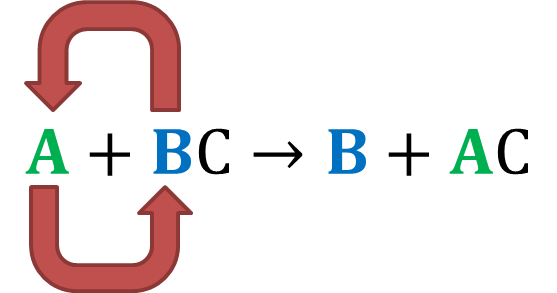
Mountain View High School Unit 111 Chemical Reactions
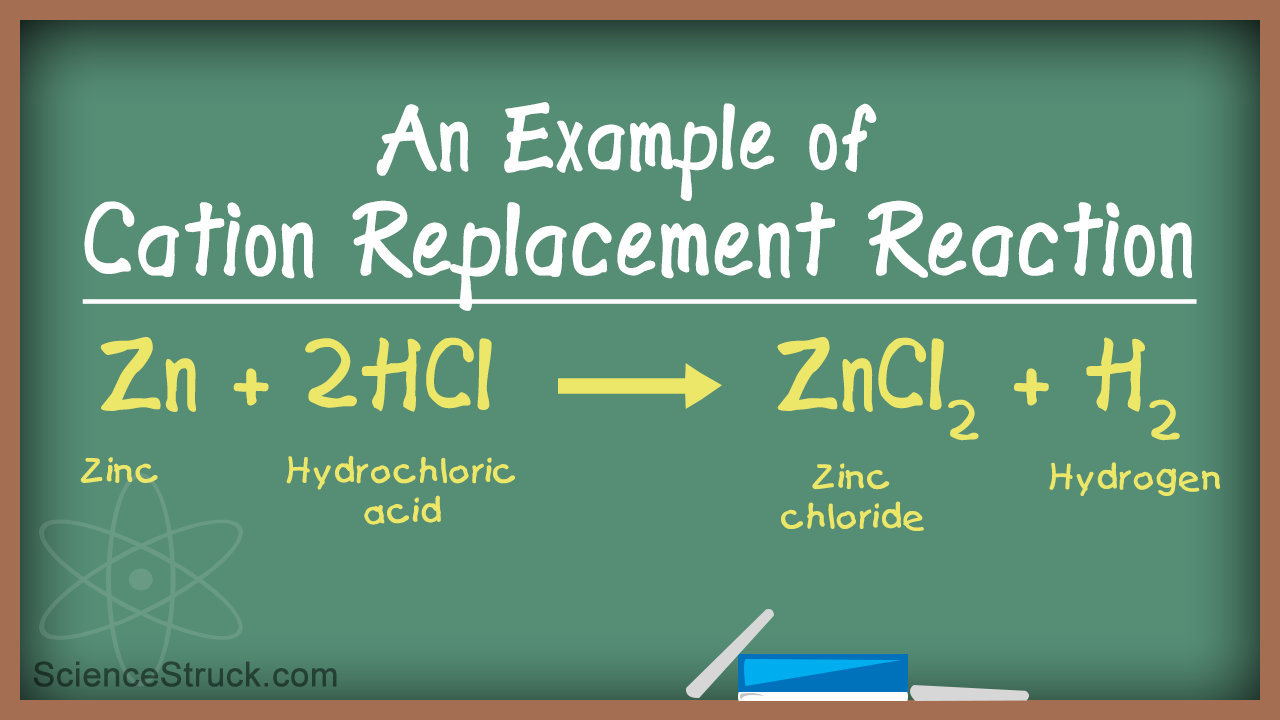
Examples Of Single Replacement Reactions Science Struck

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
0 comments
Post a Comment